Abbott's i-STAT TBI cartridge has received clearance from the U.S. Food and Drug Administration (FDA) to be used with whole blood, allowing doctors to help assess patients with suspected concussion at the patient's bedside and obtain lab-quality results in 15 minutes. Previously, the tests to help with the assessment of TBI were only cleared for use with plasma or serum, requiring samples to be sent to a lab for processing and testing.
News
September 22, 2022
BARDA is partnering with Abbott to broaden the company’s current traumatic brain injury (TBI) test that will aid healthcare providers in diagnosing and determining severity of TBI in adults and children.
August 11, 2022
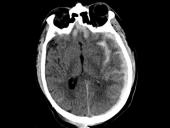
Blood tests taken within 24 hours of a traumatic brain injury (TBI) flag which patients are likely to die and which patients are likely to survive with severe disability, according to a study headed by UC San Francisco, the University of Pennsylvania and the University of Michigan. Their results – available within minutes – may confirm the need for prompt surgical interventions or may help guide conversations with families in cases of devastating injury.